
Introduction
The sensitivity and toxic and side effects of 51 targeted drugs (including 34 approved drugs and 17 investigated drugs), as well as 9 commonly used chemotherapy drugs in colorectal cancer treatment regimens are precisely and accurately interpreted, which assist clinicians to formulate the personalized treatment regimens more accurately, and improve the survival benefit of patients.
It enables:
(1) Comprehensively include key genes of colorectal cancer, break through the limitations of conventional methods;
(2) Assist in the selection of appropriate drugs and treatment regimens, and improve treatment effects;
(3) Perform dynamic monitoring.
Detection of 10 genes related to colorectal cancer

Detection of 230 genes related to colorectal cancer

Note: The sample DNA concentration is required to be ≥10 ng/ul. Insufficient DNA concentration may lead to detection failure.
Deficient mismatch repair (dMMR) tumors are more prone to benefit from immunotherapy
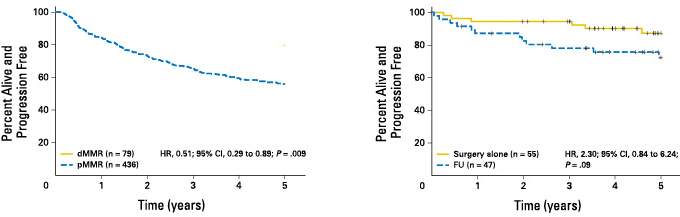
Stage II colorectal cancer patients with MSI-H have a better prognosis, and the 5-year survival rate is significantly higher than that with MSI-L/MSS[1] 。
Stage II colorectal cancer patients with MSI-H do not benefit from fluorouracil chemotherapy[1] 。
Stage II colorectal cancer patients with MSI-H do not benefit from fluorouracil chemotherapy[1] 。
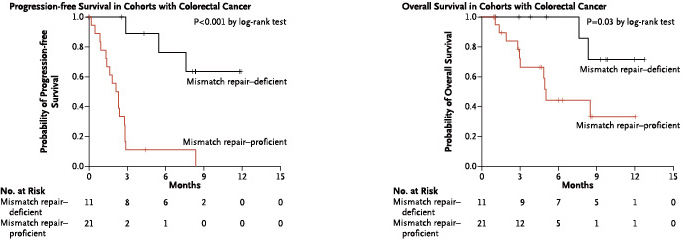
Patients with deficient mismatch repair colorectal cancer have better mPFS and mOS than patients with normal mismatch repair colorectal cancer (mPFS is only 2.2 months, mOS is 5 months), and significantly benefit from the treatment with Pembrolizumab
Reference
1、Sargent DJ, et al.Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J ClinOncol.2010 Jul 10;28(20):3219-26.
2、Le DT, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520.
1、Sargent DJ, et al.Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J ClinOncol.2010 Jul 10;28(20):3219-26.
2、Le DT, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520.






